Abstract
Introduction: Autoimmune-mediated thrombotic thrombocytopenic purpura (aTTP) is a rare and life-threatening hematologic condition. First-line therapies include plasma exchange (PLEX), corticosteroids, caplacizumab, and rituximab. Optimal immunosuppressive therapy for rituximab-refractory aTTP has not been well established. Bortezomib is a biologically appealing treatment to deplete autoantibody producing CD138-positive plasma cells, yet its evidence is limited to case reports and case series (Patriquin 2016, Ratnasingam 2016, and Maschan 2021). Thus, we sought to report our institution's bortezomib experience, with an updated systematic review to evaluate the efficacy of bortezomib in relapsed and refractory aTTP.
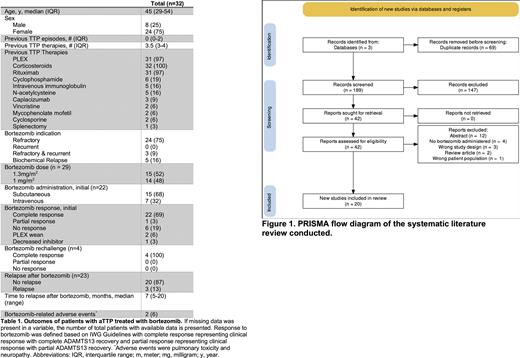
Methods: All patients at the University of Texas Southwestern Medical Center and Parkland Memorial Hospital treated with bortezomib for aTTP from 1/1/2013 to 6/1/2022 were included in this retrospective case series. A systematic review of the literature was performed in accordance with PRISMA guidelines to identify patients with aTTP treated with bortezomib. A search of MEDLINE, Embase, and Web of Science was performed. Articles were independently reviewed by two reviewers (N.L. and N.B.) by title and abstract followed by a complete paper review using Covidence systematic review software (Melbourne, Australia). Disagreements regarding inclusion were decided by joint decision. Patient demographics and clinical data were extracted from eligible studies and recorded by one reviewer (N.L.) All analyses were performed in STATA 14 (College Station, TX). We defined outcomes of clinical response based on the international working consensus report (Cuker 2021) for clinical remission for our cohort and case reports where sufficient information was available. If insufficient information was available, the authors' clinical judgment was utilized.
Results: Our retrospective institutional review yielded eight patients. The literature search identified 258 articles with 189 unique articles that were screened, identifying 27 patients from 20 peer-reviewed publications. Of the combined 35 patients, two of the patients at our center were previously reported in the literature and one patient was reported twice, resulting in 32 unique patients. Patients receiving bortezomib therapy had a median age of 45 and a female predominance (75%). Patients received a median of 3.5 therapies before initiation of bortezomib. Indications for bortezomib therapy included refractory disease in the initial presentation (n=24, 75%), refractory disease in a recurrent presentation (n=3, 9%), or biochemical relapse (n=5, 16%). Twenty-two patients (69%) treated with bortezomib achieved a complete response to the initial cycle of bortezomib, with one patient achieving a partial response. Bortezomib was dosed at either 1.3 mg/m2 (n=15, 52%) or 1 mg/m2 (n=14, 48%) and more frequently administered subcutaneously (68%) than intravenously (32%). At our institution (n=8), patients received either weekly doses for 4 weeks (n=6), day 1 and 8 every 21 days (n=1), or day 1, 8, and 15 every 28 days (n=1) with a total median dose of 5.2 mg/m2 (minimum of 4 mg/m2 to maximum of 19.5 mg/m2). Four patients (out of 32) were rechallenged with bortezomib, and all attained a complete response. Of the patients with clinical response to bortezomib and documentation of a clinical course (n=23), 87% (20 out of 23 patients) remained in remission with a median follow-up of 12 months (range: 1-99 months). Median time to relapse was 7 months (n=3, 13%; range: 5-20 months). Two patients (n= 2 out of 32; 6%) had adverse events, including pulmonary toxicity and neuropathy.
Discussion: Bortezomib demonstrates efficacy in some patients with relapsed and/or refractory aTTP. Preemptive bortezomib may also prevent clinical relapses in patients with biochemical relapse. Our study is susceptible to reporting bias in the literature, and the use of bortezomib is currently off-label. Given bortezomib's ease of administration, short duration of administration, and favorable safety profile, its use in relapsed and/or refractory aTTP is an attractive therapy worthy of further study.
Disclosures
Sarode:VarmX: Consultancy; Octapharma: Consultancy; CSL Behring: Consultancy; Takeda: Research Funding; Siemens: Research Funding; Cerus: Research Funding.
OffLabel Disclosure:
Bortezomib depletes CD138+ plasma cells that are involved in autoantibody production in iTTP. It has been used successfully off-label for refractory autoimmune TTP.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal